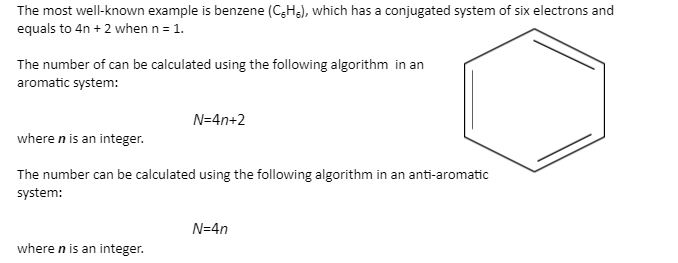
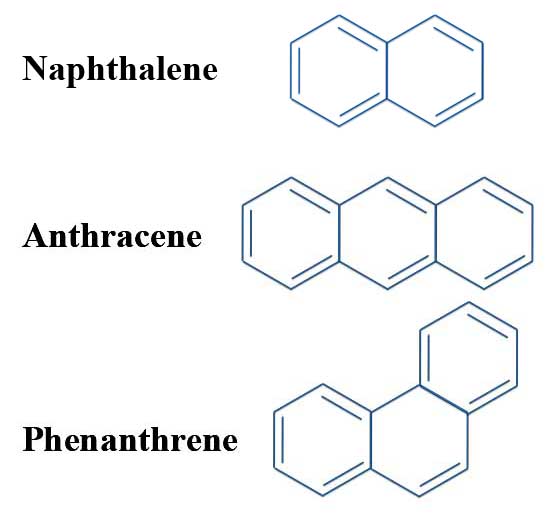
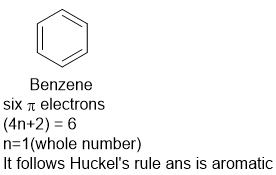
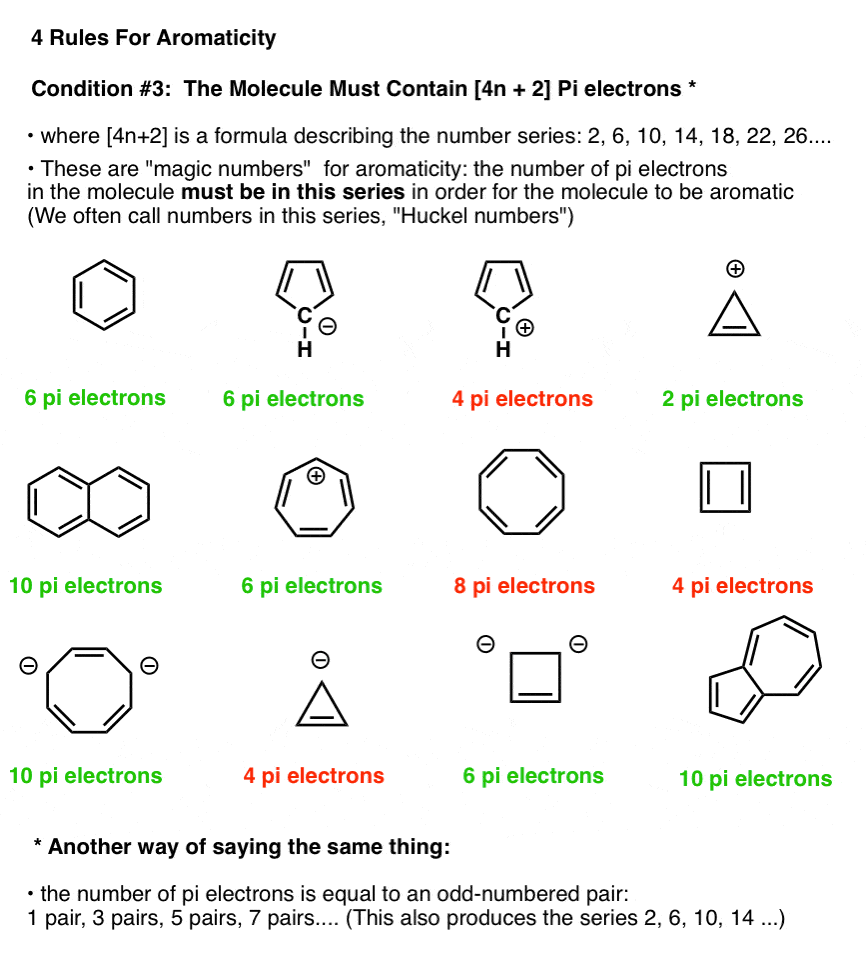
√100以上 4n 2 Rule Benzene 112551-Benzene 4n+2 Rule
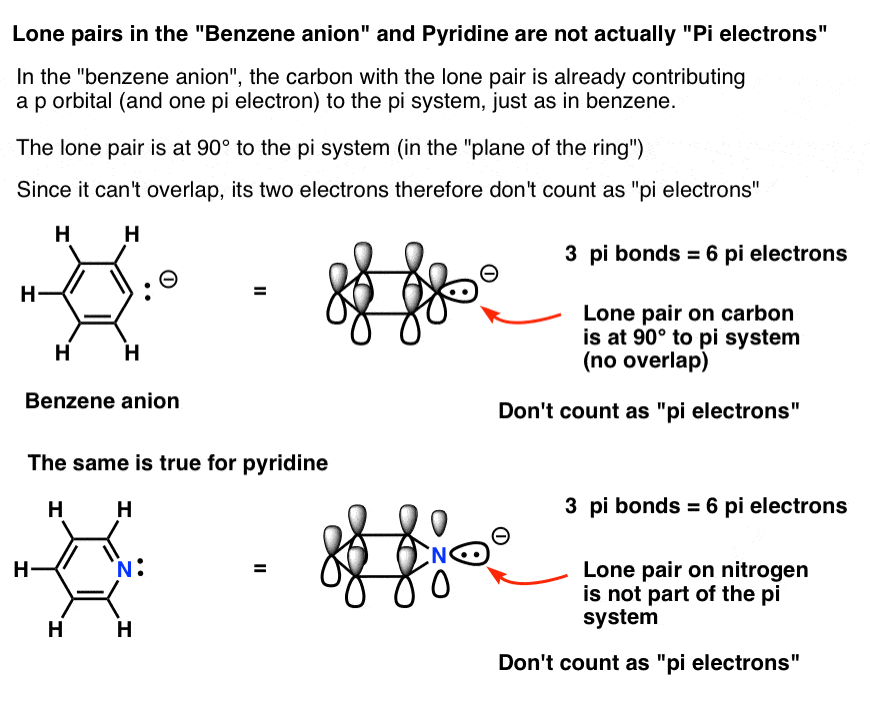
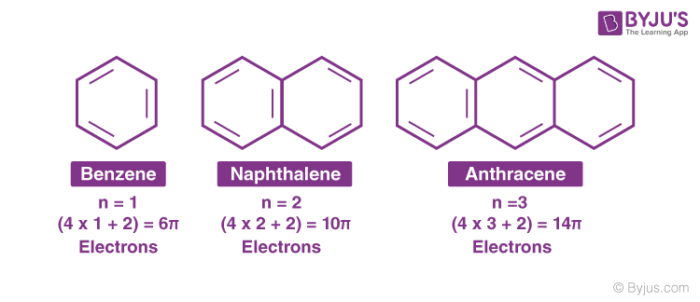
Therefore, the K(4n 2) rule is of rather limited range and the table given in Ref 1 reduces Fig 1 Schlegel diagrams of buckminsterfullerene (top diagram) and four of its isomers The presence of 4n 2 π Electrons are a must in all planar Aromatic Compounds where n123 It is antiaromatic if all of this is correct except it has 4n electrons Any deviation from theseThus, pyridine has 6 pi – electrons and if we keep n= 1 in Huckel's 4n 2 rule then 412 = 6 So, pyridine follows Huckel's rule and fulfils other
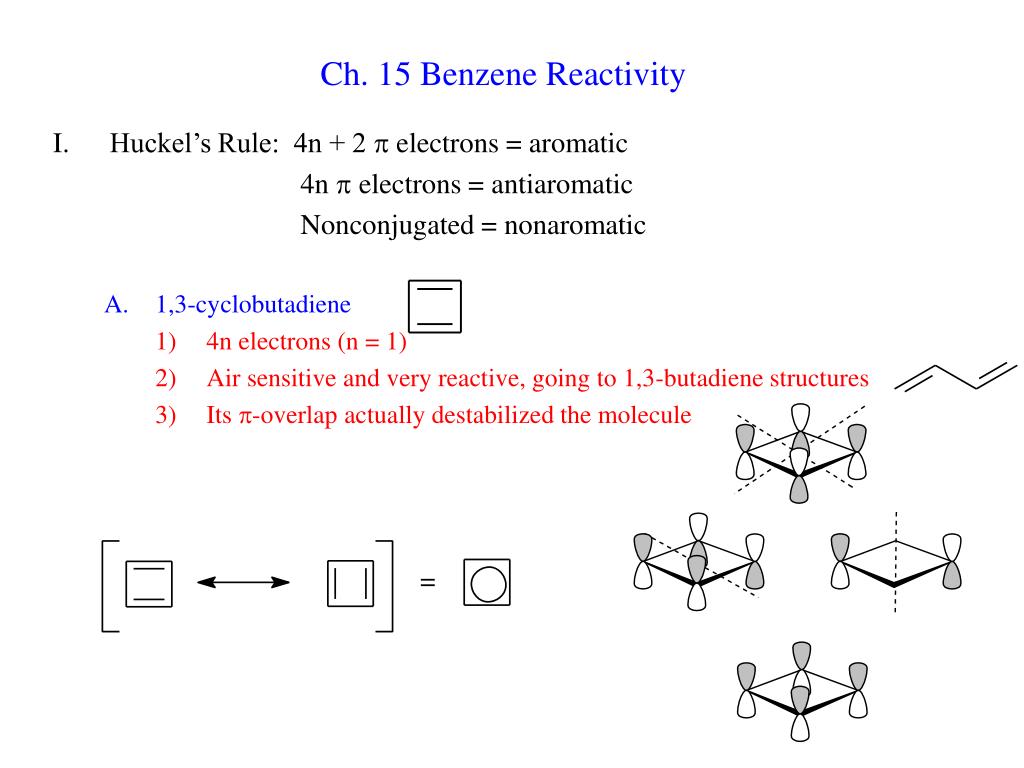
Ppt Ch 15 Benzene Reactivity Powerpoint Presentation Free Download Id
Benzene 4n+2 rule
Benzene 4n+2 rule-The planar geometries sustain strong diamagnetic ring current comparable with that of benzene In contrast, the calculated multicenter normalized Giambiagi electron delocalization index I NG To apply the 4n2 rule, first count the number of π electrons in the molecule Then, set this number equal to 4n2 and solve for n If is 0 or any positive integer (1, 2, 3,), the rule




Baird Aromaticity In Excited States And Open Shell Ground States Sciencedirect
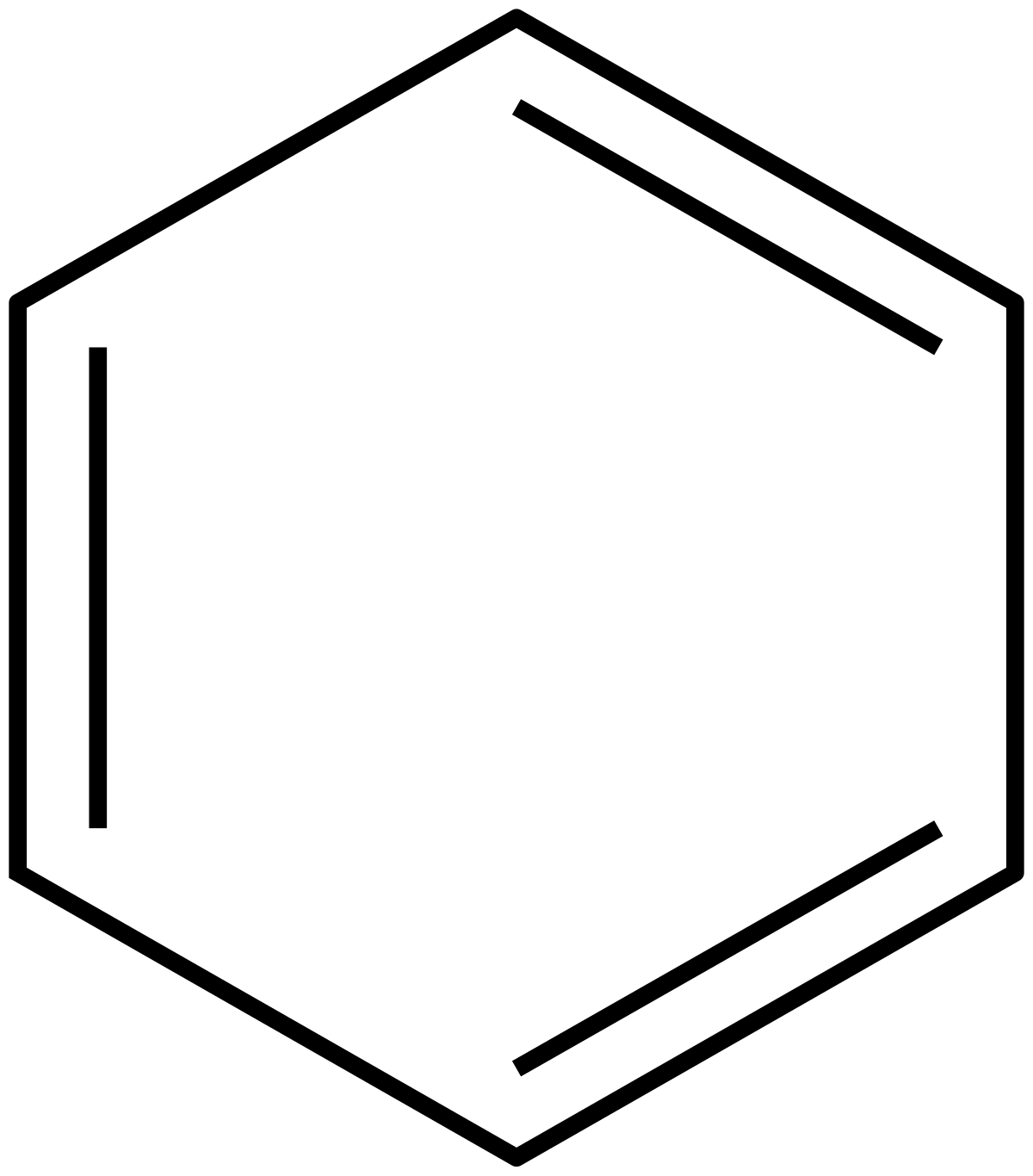
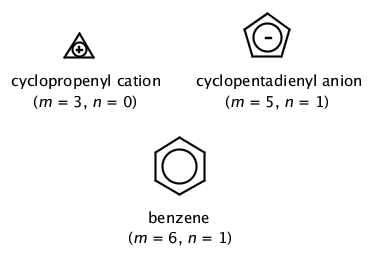
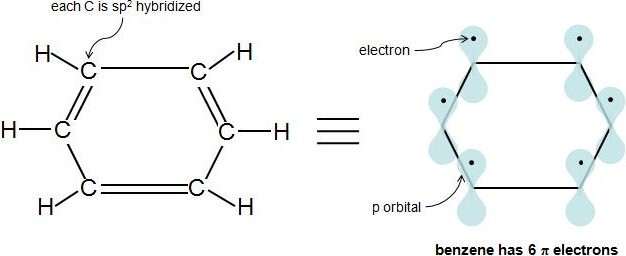
Benzene has three CC double bonds, which mean that we have 3 pairs of π electrons = 6 π electrons = a 4n2 numberWhat is N in the 4n2 rule?It s known as Huckel s rule, or the 4n 2 rule To apply this rule, begin by assigning 4n 2 = number of electrons in a cyclic system Next, solve for n, and if n is an integer (a whole number
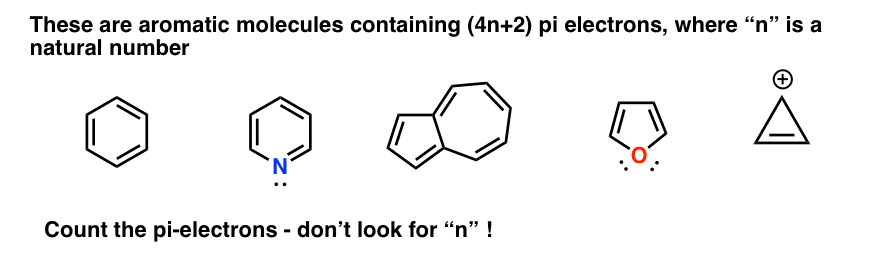
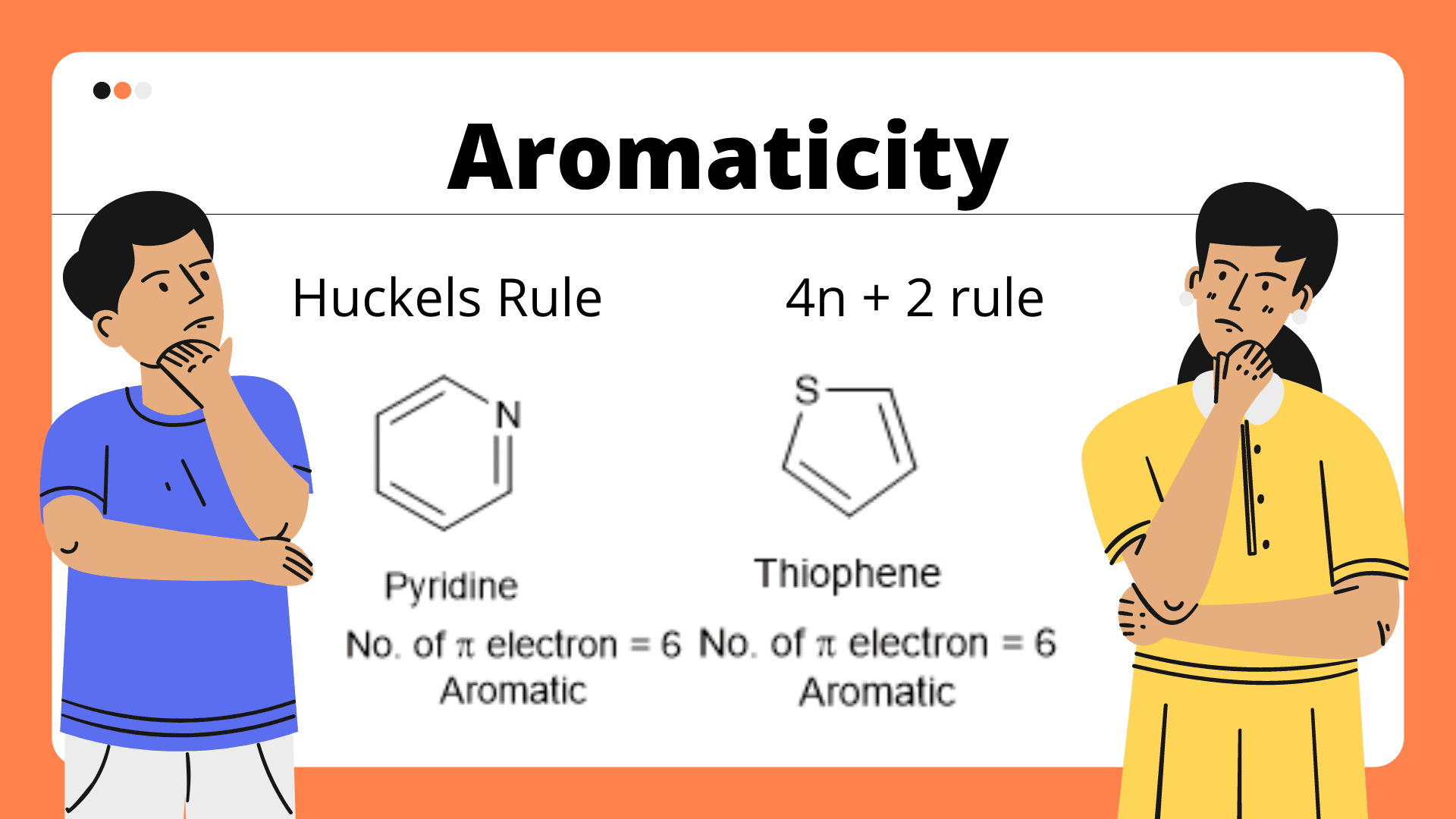
This closed‐shell or same‐spin half‐filled electronic structure provides an extra stabilization and it is the origin of several rules of aromaticity such as the Hückel 4N 2 rule,To apply the 4n2 rule, first count the number of π electrons in the molecule Then, set this number equal to 4n2 and solve for n If is 0 or any positive integer (1, 2, 3,), the rule has been metHuckle's rule It states that if a cyclic, planar molecule has (4n2) pi electrons, it is considered aromatic
Benzene (C 6 H 6) is a well known aromatic compound having 6 pi electrons (4n2 rule, n=1) Benzene has total six carbon and six hydrogen atoms with alternating single and double bonds To apply the 4n2 rule, first count the number of π electrons in the molecule Then, set this number equal to 4n2 and solve for n If is 0 or any positive integer (1, 2, 3,), the ruleTo apply the 4n2 rule, first count the number of π electrons in the molecule Then, set this number equal to 4n2 and solve for n If is 0 or any positive integer (1, 2, 3,), the rule has been met
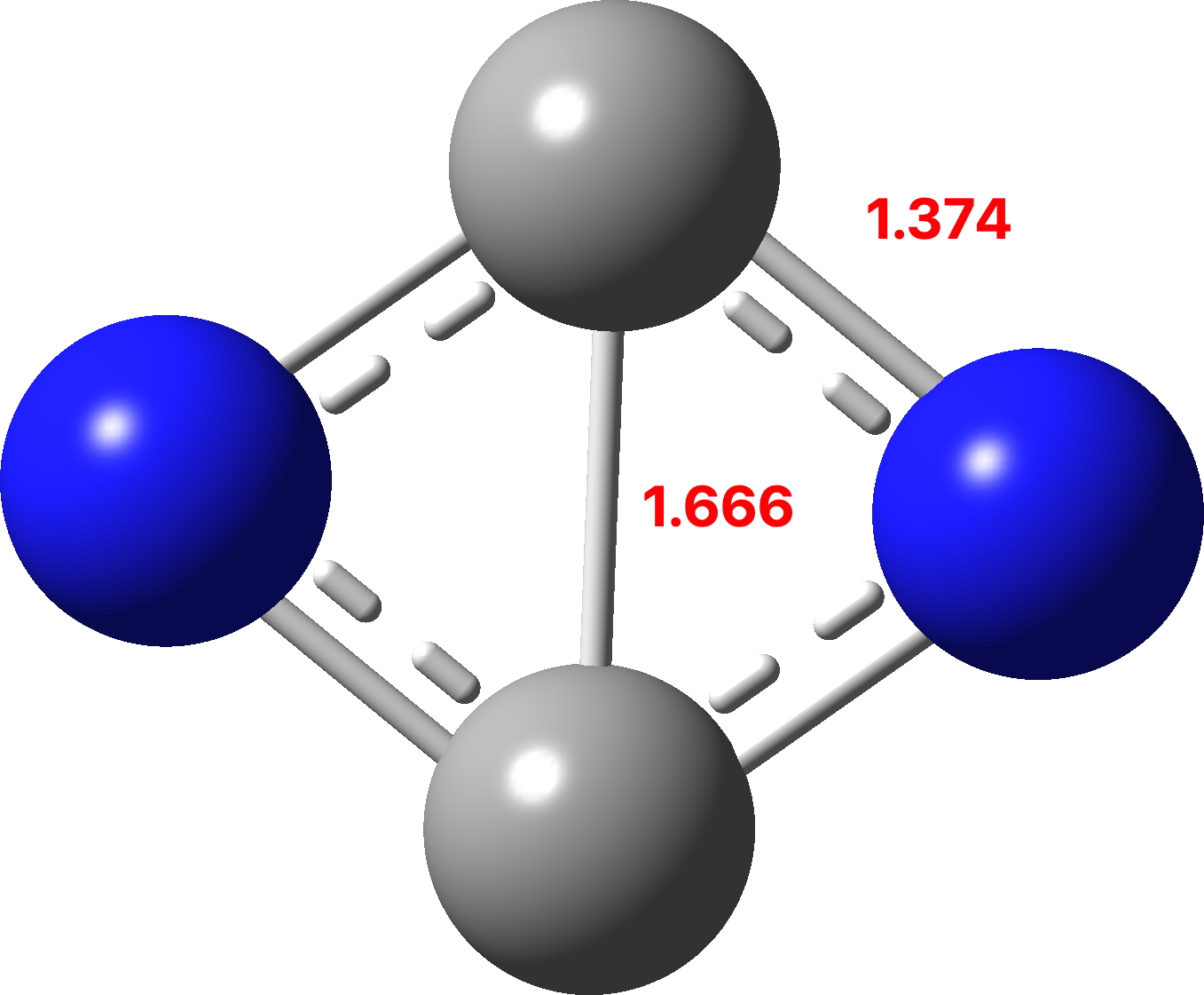



C2n2 A 10 Electron Four Atom Molecule Displaying Both Huckel 4n 2 And Baird 4n Selection Rules For Ring Aromaticity Henry Rzepa S Blog




Mobius Aromaticity Wikipedia
In 4n 2, if we put n = 1 then (4×1)2 = 6, thus it obeys Huckel’s Rule Benzene is an Aromatic Compound and possesses Aromaticity n = 1 (4×1)2 = 6π electrons Aromatic Compound OnClick here👆to get an answer to your question ️ Huckel's Rule (4n 2) i electronsThe molecule has 4n2 ∏ electrons (n=0 or any positive integer) EXAMPLE 1 BENZENE Confirm that benzene is aromatic SOLUTION To apply the 4n2 rule, first count the number of π



Q Tbn And9gcqeccqqcejialwbx5avq U0kre0ap7r70 Fynmggh5 Qmjxc3nh0m N Usqp Cau
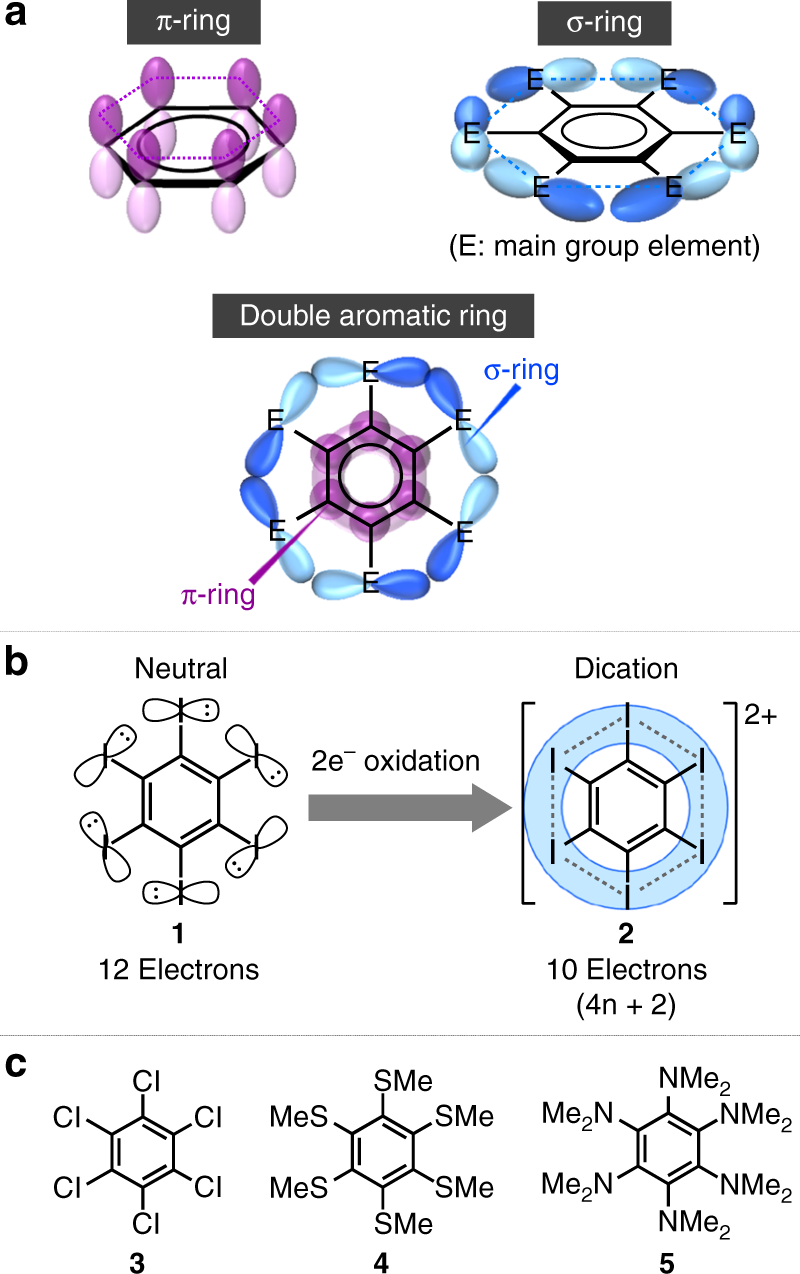



Double Aromaticity Arising From S And P Rings Communications Chemistry
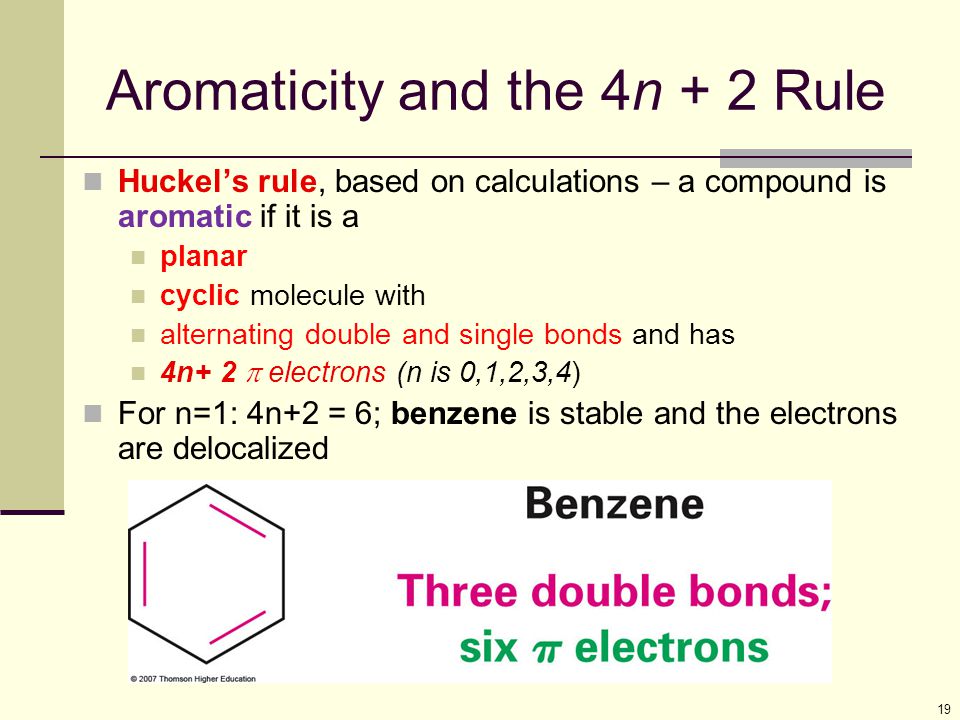
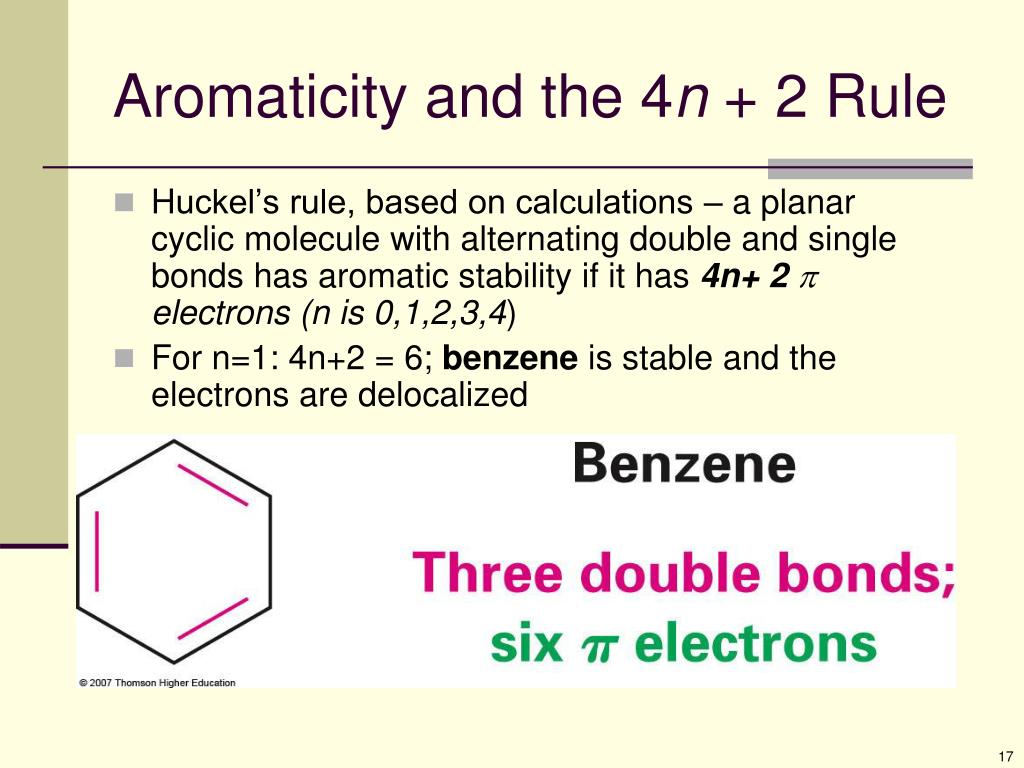
Aromaticity and the Hückel 4n 2 Rule Benzene and other benzenelike aromatic molecules have several things in common like I Benzene is cyclic and conjugated Aromatic compounds containAromatic compounds contain 4n2 π electrons, where n is a whole number starting from 0 This is called the Hückel’s rule discovered by Erich Hückel in 1931 For example, Benzene has 6 π#alchemistsonia #benzene #aromaticity #aromaticcompounds #huckelrule #organicchemistry #organicchemistrynotes



Illustrated Glossary Of Organic Chemistry Term
.jpg?revision=1&size=bestfit&width=440&height=181)



15 3 Aromaticity And The Huckel 4n 2 Rule Chemistry Libretexts
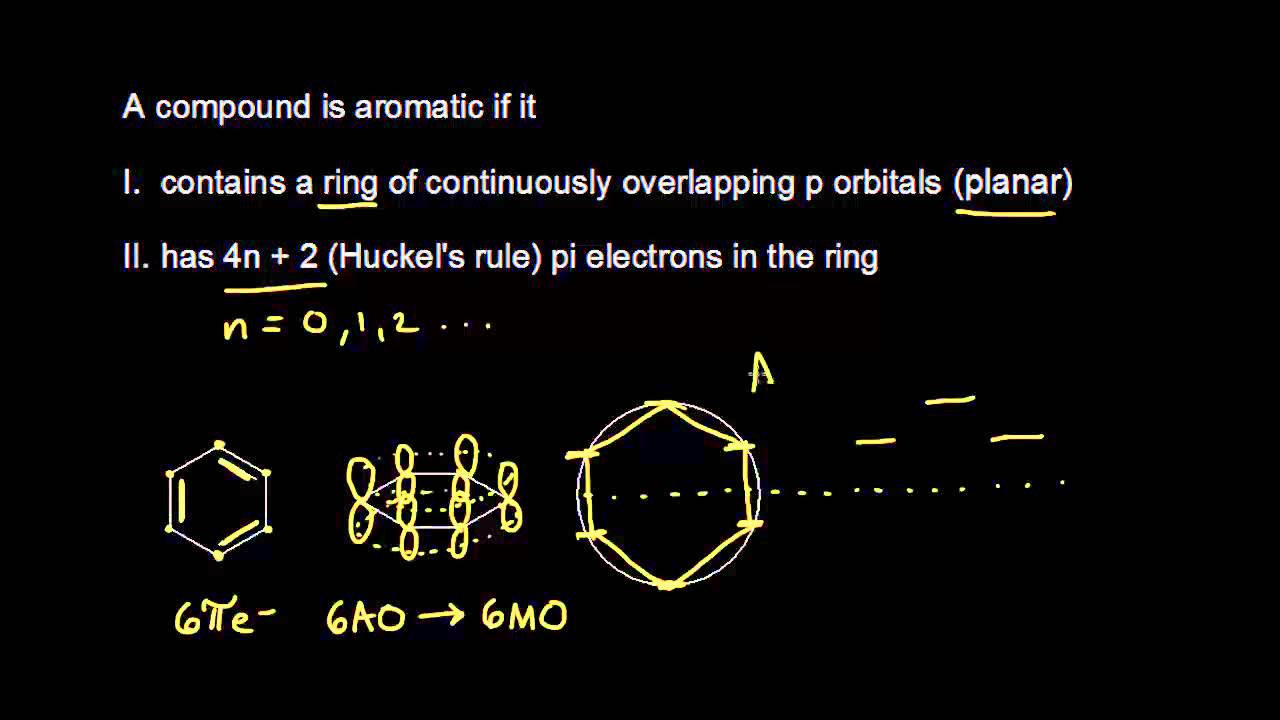
Benzene Cyclo octatetraene (4×1)2 = 6 ≠ 8 pielectrons in Cyclo octatetraene Hence, Cyclo octatetraene is not an aromatic compound and does not possess aromaticity In addition, the 4n 2 rule has been used to rationalize multifold aromaticity in allmetal clusters 3 More recently, Mayer derived the 4n 2 rule analytically to determine theThe (4n2) rule is a consequence of the degeneracy of the π orbitals in cyclic conjugated hydrocarbon molecules As predicted by Hückel molecular orbital theory, the lowest π orbital in




Huckel S Rule What Does 4n 2 Mean Master Organic Chemistry




Huckel S Rule For Aromaticity Time Saving Shortcut Youtube
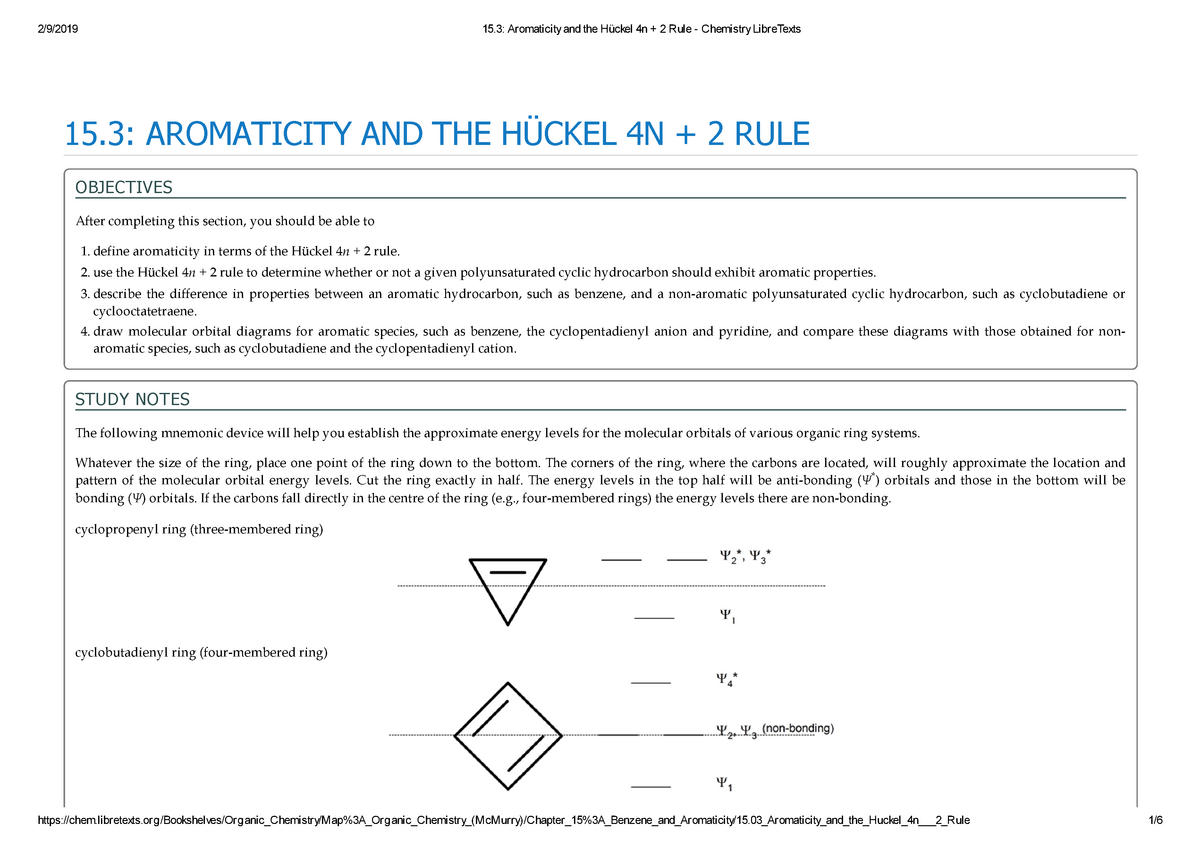
OBJECTIVES After completing this section, you should be able to 1 define aromaticity in terms of the Hückel 4 n 2 rule 2 use the Hückel 4 n 2 rule to determine whether or not a givenTo apply this rule, begin by assigning 4n 2 = number of 7 electrons in a cyclic system Next, solve for n, and if n is an integer (a whole number ), the system is aromatic In the case of benzene, 4n If is 0 or any positive integer (1, 2, 3,), the rule has been metFor example, benzene has sixπ electrons 4n 2 = π 4n 2 = 6 4n 2 = 6 4n = 62 4n = 4 n = 4/4 n = 1 For




Chemistry Huckel S Rule 4n 2 Rule In Order To Be Aromatic A Molecule Must Have A Certain Number Of Pi Electrons Electrons With Pi Bonds Or Lone Pairs Within P Orbitals Within
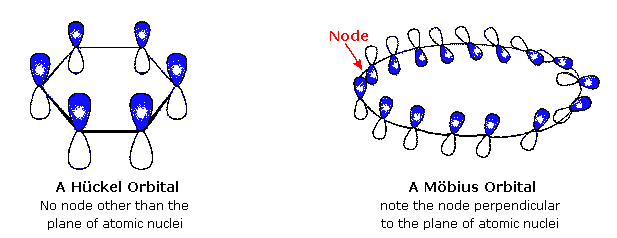



A Mobius 16 Pi Electron System
Benzene is an aromatic hydrocarbon because it obeys Hückel’s rule Originally, benzene was considered aromatic because of its smell it has an “aromatic” odor It is now Does pyridine follow 4n 2 rule?This organic chemistry video tutorial shows you how to tell if a compound is aromatic, antiaromatic or nonaromatic by using huckel's rule / number of 4n2 pi electrons, and features of the




In Huckel S 4n 2 Pi Rule For Aromaticity N Represents




Huckel S Rule Definition Formula And Examples
Benzene is particularly stable (=aromatic) because the cyclically conjugated π electrons all occupy stable doublyfilled molecular orbitals for which the total πelectron count conforms to theAccording to Huckel's Rule, you can determine if a molecule is aromatic, antiaromatic, or nonaromatic by using the number of π electrons (N) and the physical structure of the ring In the comment bar, you had said that, it is for benzene, right!




15 Benzene And Aromaticity Ppt Video Online Download



Q Tbn And9gcqomlffumwe72vykpnhdvka2cvhfhhmfemoneqe4x8z Wqgltffdfto Usqp Cau
To apply the 4n2 rule, first count the number of π electrons in the molecule Then, set this number equal to 4n2 and solve for n If is 0 or any positive integer (1, 2, 3,), the rule has been metFor some reason none of my organic chemistry textbooks, lectures, etc explain what N actually is, so I made this videoQ The 4n2 Rule is also sometimes called the Huckel Rule Recall that n can be 0,1,2,3, so that the systems which are highly stabilized have 2,6,10,14,pi electrons in a cyclic conjugated
.jpg?revision=1&size=bestfit&width=317&height=292)



15 3 Aromaticity And The Huckel 4n 2 Rule Chemistry Libretexts
.jpg?revision=1)



17 5 Aromaticity And Huckel S Rule Chemistry Libretexts
• These criteria are collectively called Hückel’s 4n2 rule or the 4n2 rule 52 157 Introduction to Aromatic Compounds 6 p electrons n=1 The EPM shows the high electron density (red) above Before using the 4n2 rule, count the number of electrons in the molecule Set this number to 4n2 to find n If it is 0, or any other positive integer, the condition is met (1, 2, 3,View Huckel's Rule ABOUT BENZENEdocx from CHE ORGANIC CH at Govt College, Taunsa Sharif (DG Khan) Huckel's Rule Aromatic, Antiaromatic, and Nonaromatic In 1931
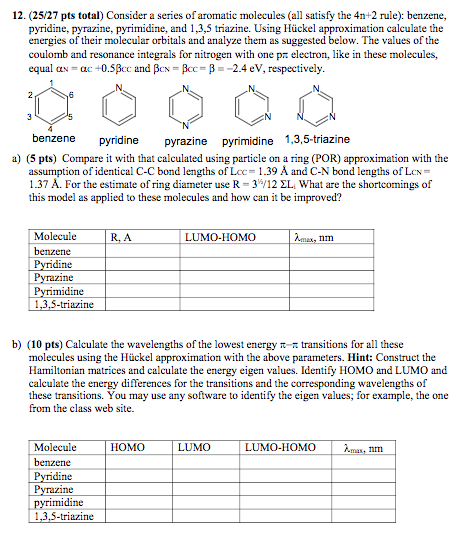



12 25 27 Pts Total Consider A Series Of Aromatic Chegg Com



Rules For Aromaticity The 4 Key Factors Master Organic Chemistry
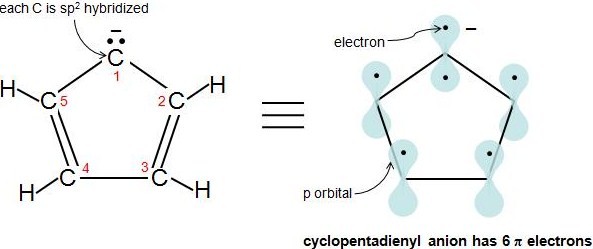
Application of Resonance and of the \(4n 2\) Rule to Cyclic Ions The hydrogens of the \(\ce{CH_2}\) group of 1,3cyclopentadiene are acidic In fact, they are considerablyLet me explain InHuckel's rule can be used to determine which one will be aromatic The structure of benzene shows that it has 6 π electrons If we put n = 1 in 4xn 2, we get (4x1)2 = 6, which follows




Huckel S Rule Aromatic And Antiaromatic Compounds Chemistry Steps
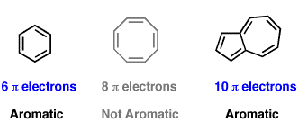



Huckel S Rule What Does 4n 2 Mean Master Organic Chemistry
2 In the 4n2 Rule (“Huckel’s Rule”), “n” Is Not A Characteristic Of The Molecule! The 14 πelectron bridged annulene on the right is an aromatic 4n 2 system and has the same anisotropy as benzene It must have 4n2 𝛑 electrons The statistical distribution of spinsAbout Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How works Test new features Press Copyright Contact us Creators




Huckel S Rule Definition Formula And Examples




Ppt Ch 15 Benzene Reactivity Powerpoint Presentation Free Download Id
In 4n 2, if we put n = 1 then (4×1)2 = 6, thus it obeys Huckel’s Rule Benzene is an Aromatic Compound and possesses Aromaticity n = 1 (4×1)2 = 6π electrons Aromatic Compound OnAnswer (1 of 6) The classical answer will be via Frost Circles, which give the MOs for Huckel and Mobius systems Suzanka has given a fantastic answer from that perspective and I have nothing1 153 AROMATICITY AND THE HUCKEL 4N 2 RULE Objectives After completing this section, you should be able to 1 define aromaticity in terms of the Hückel 4 n 2 rule 2 use the



15 Benzene And Aromaticity Ppt Video Online Download



Huckel S Rule What Does 4n 2 Mean Master Organic Chemistry
“n” is not a characteristic of the molecule!Aromatic compounds exhibit magnetically induced ring current, but the reverse conclusion that magnetically induced ring current identifies aromaticity is not justified The 4n2 rule as Benzene is an example of an aromatic compound There are three π bonds and thus total number of electrons = 3×2=6 which is a multiple of (4n2) where n=1 What is 4n 2pi?



Despite Having A 4n Pi Electron System Why Is Cyclopentadiene Non Aromatic Quora



15 3 Aromaticity And The Huckel 4n 2 Rule Chemistry Libre Texts 15 Aromaticity And The Huckel Studocu




Baird Aromaticity In Excited States And Open Shell Ground States Sciencedirect



Huckel S Rule Wikipedia



Notes On Huckel S Rule Unacademy




130 What Is So Special About 4n 2 P Electrons Madoverchemistry



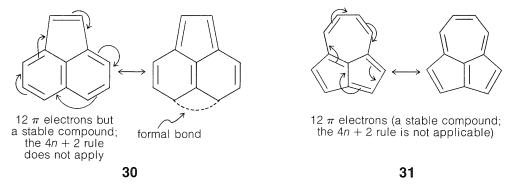
The Problem With Pyrene Michael J S Dewar To The Rescue
.png?revision=1)



15 4 Aromatic Ions Chemistry Libretexts



Aromaticity Rules 4n 2 Rule Chemistry Notes



Rules For Aromaticity The 4 Key Factors Master Organic Chemistry




It Seems Like Kekulene Is Considered Aromatic Despite Not Following The 4n 2 Rule 48 Pi Electrons Out Of Curiosity Does Anyone Know Why This Is I Assume Aromaticity Gets Complicated As The
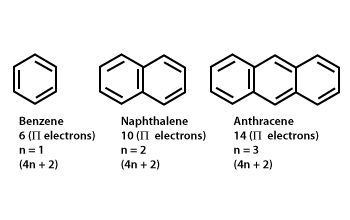



Aromaticity




2 Could The Particle On A Ring Model Be Used To Chegg Com




Analysis Of Huckel S 4n 2 Rule Through Electronic Delocalization Measures The Journal Of Physical Chemistry A



Aromaticity Rules And Definition
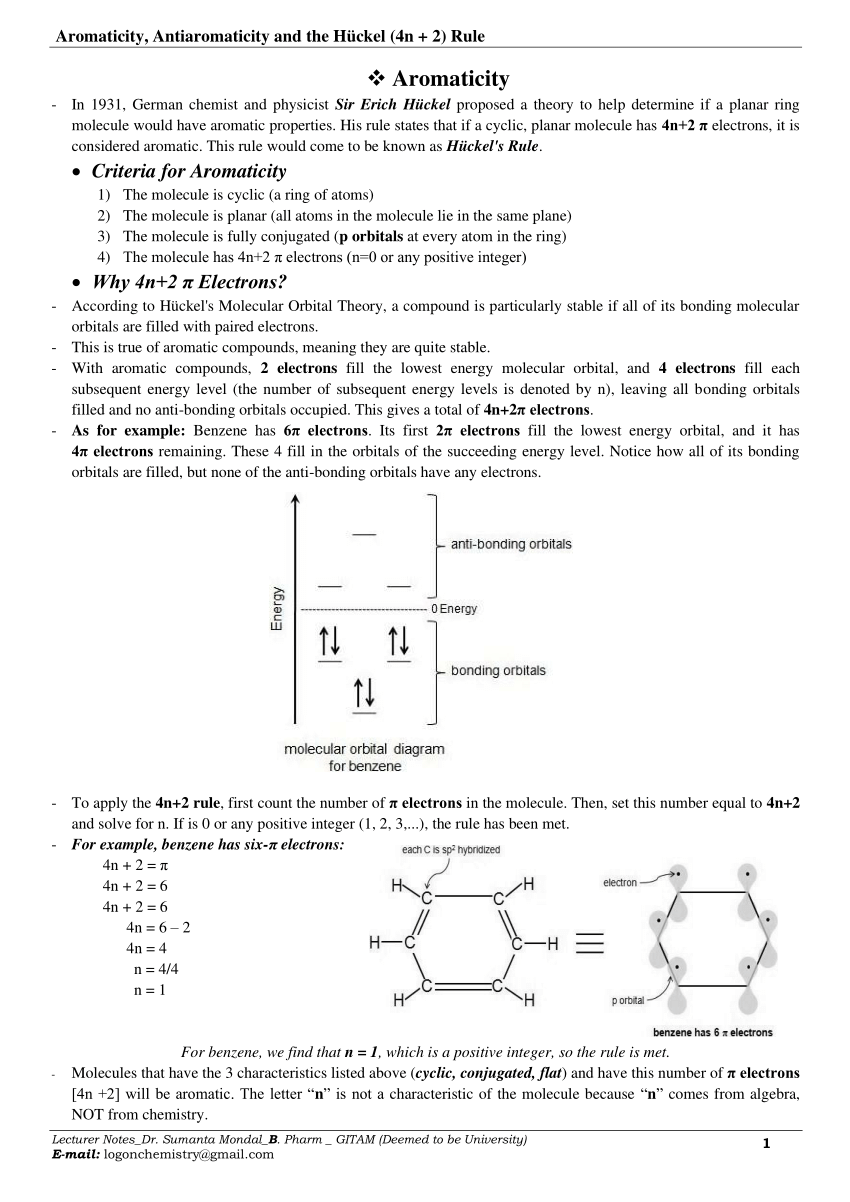



Pdf Aromaticity Antiaromaticity Homoaromaticity And The Huckel 4n 2 Rule
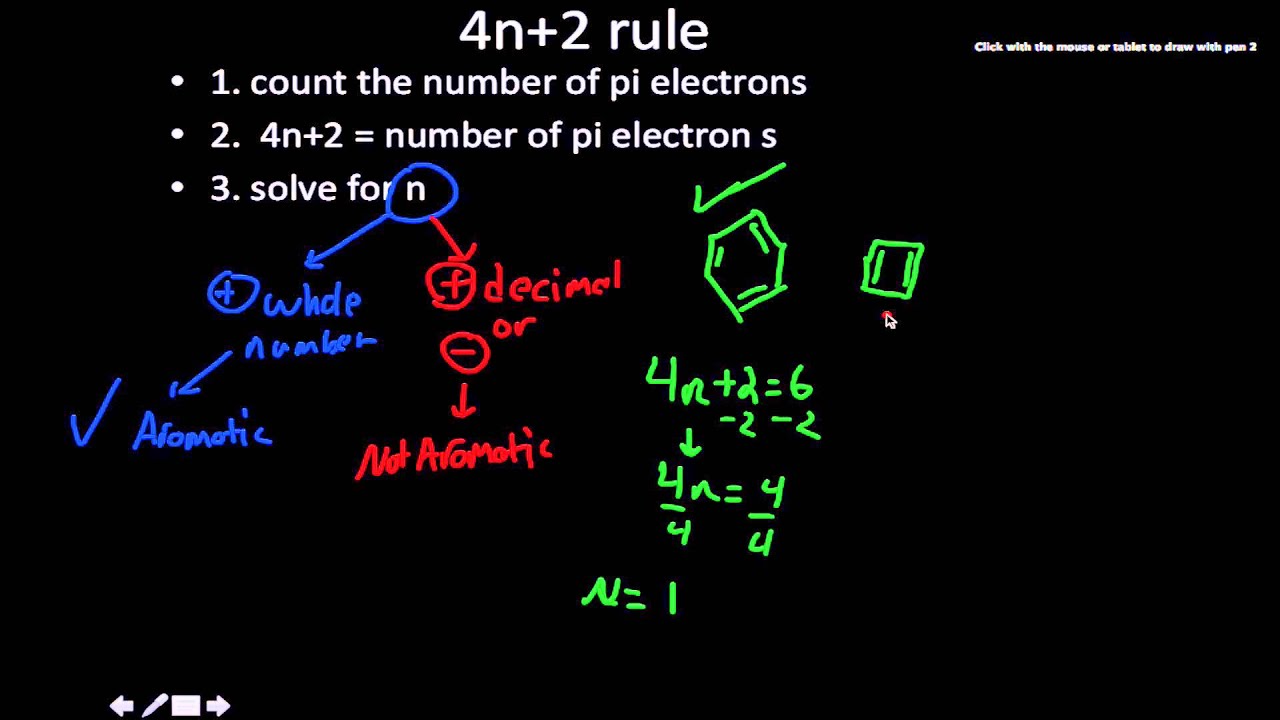



4n 2 Rule Youtube




Aromaticity The Huckel 4 N 2 Rule And Magnetic Current Zhao 17 Chemistryselect Wiley Online Library
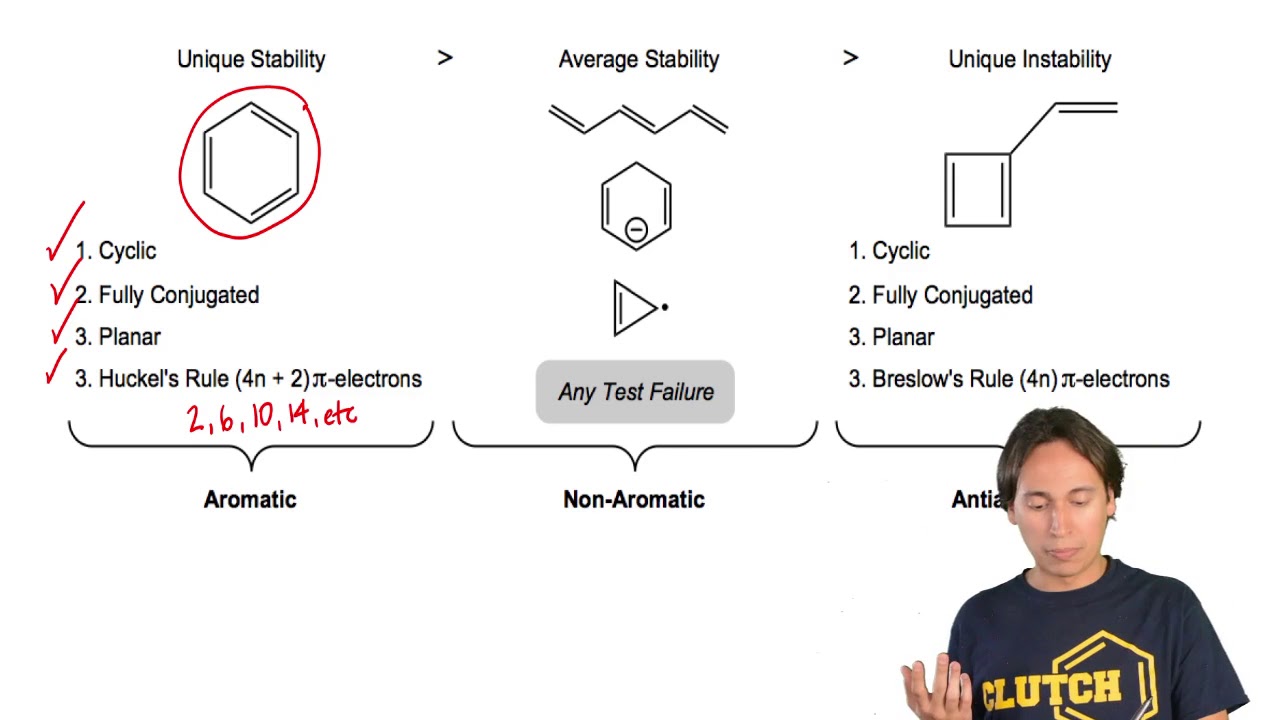



Huckel S Rule Organic Chemistry Video Clutch Prep



Iupac Huckel 4 Em N Em 2 Rule H



Aromatic Antiaromatic Or Nonaromatic Huckel S Rule 4n 2 Heterocycles Youtube



How Is Anthracene Aromatic Quora



Aromaticity Rules 4n 2 Rule Chemistry Notes




Ch 15 Benzene Reactivity Huckel S Rule 4n 2 P Electrons Aromatic Ppt Video Online Download



Aromatic Stability I Video Khan Academy
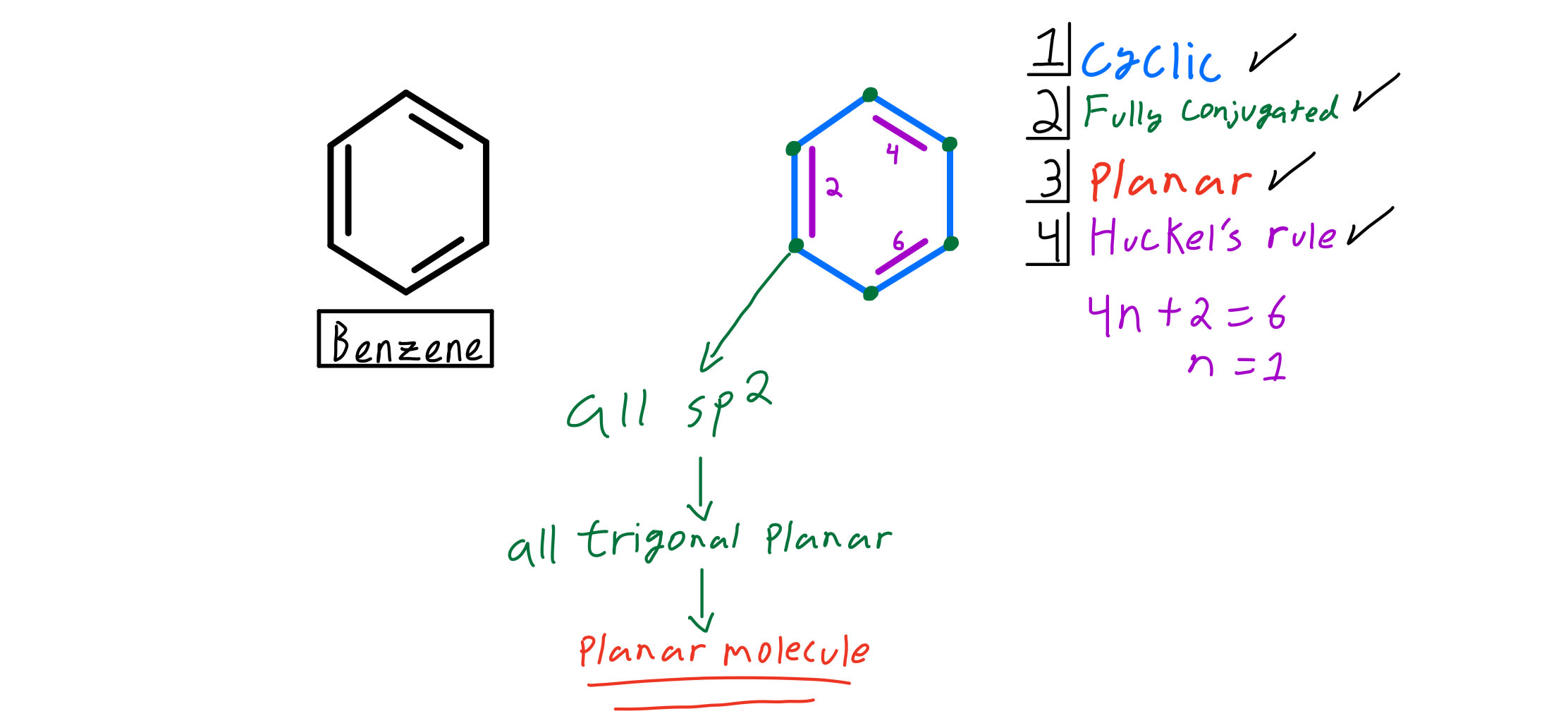



Huckel S Rule Organic Chemistry Video Clutch Prep




In Huckels Rule 4n 2 Electrons For Aromatic Compounds What Does This N Chemistry Haloalkanes And Haloarenes Meritnation Com
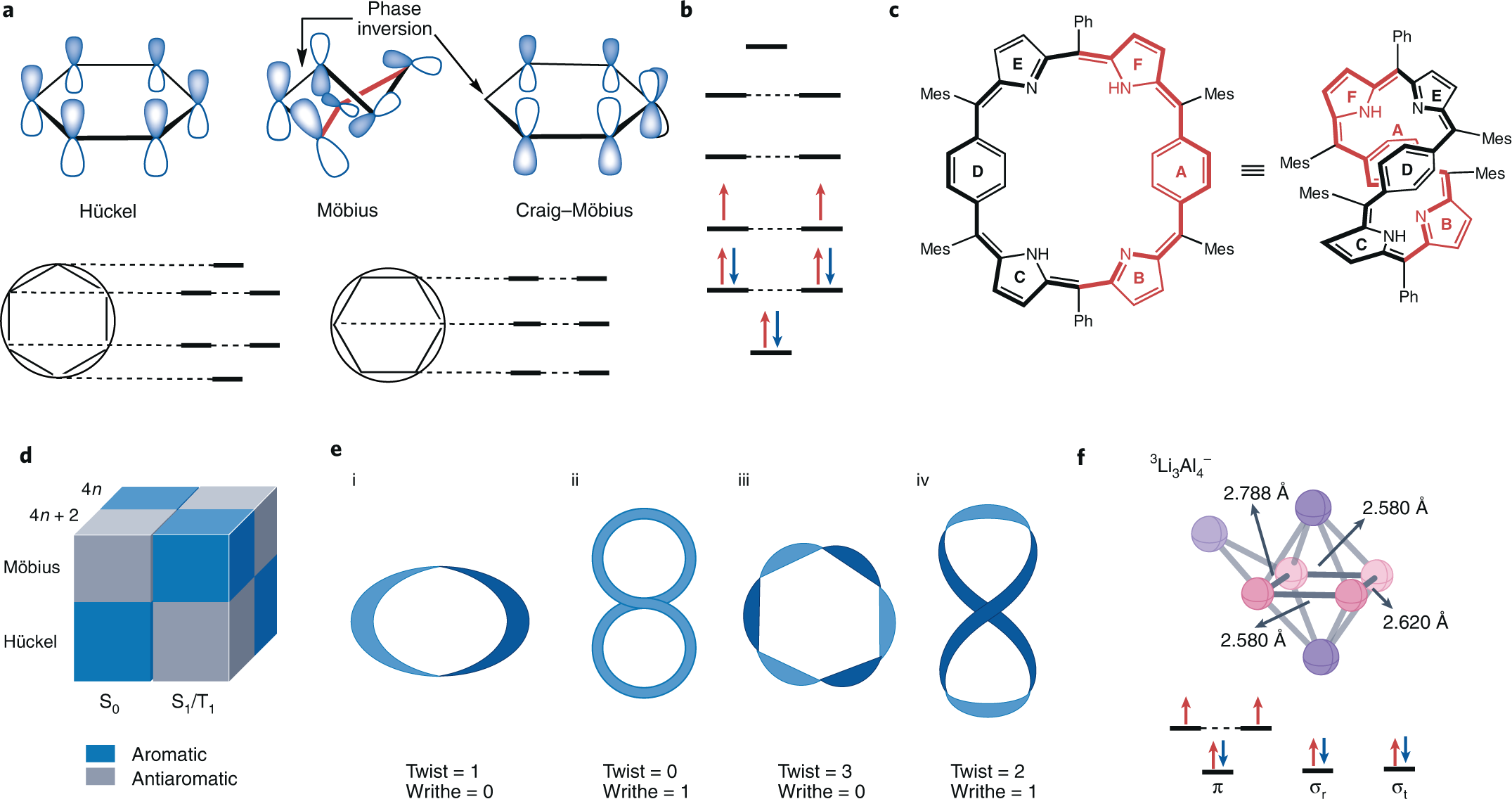



Aromaticity Rules Nature Chemistry



Why Is Cyclooctatetraene A Non Aromatic Compound Quora




Aromatic Antiaromatic Or Nonaromatic Compounds Chemistry Steps



2
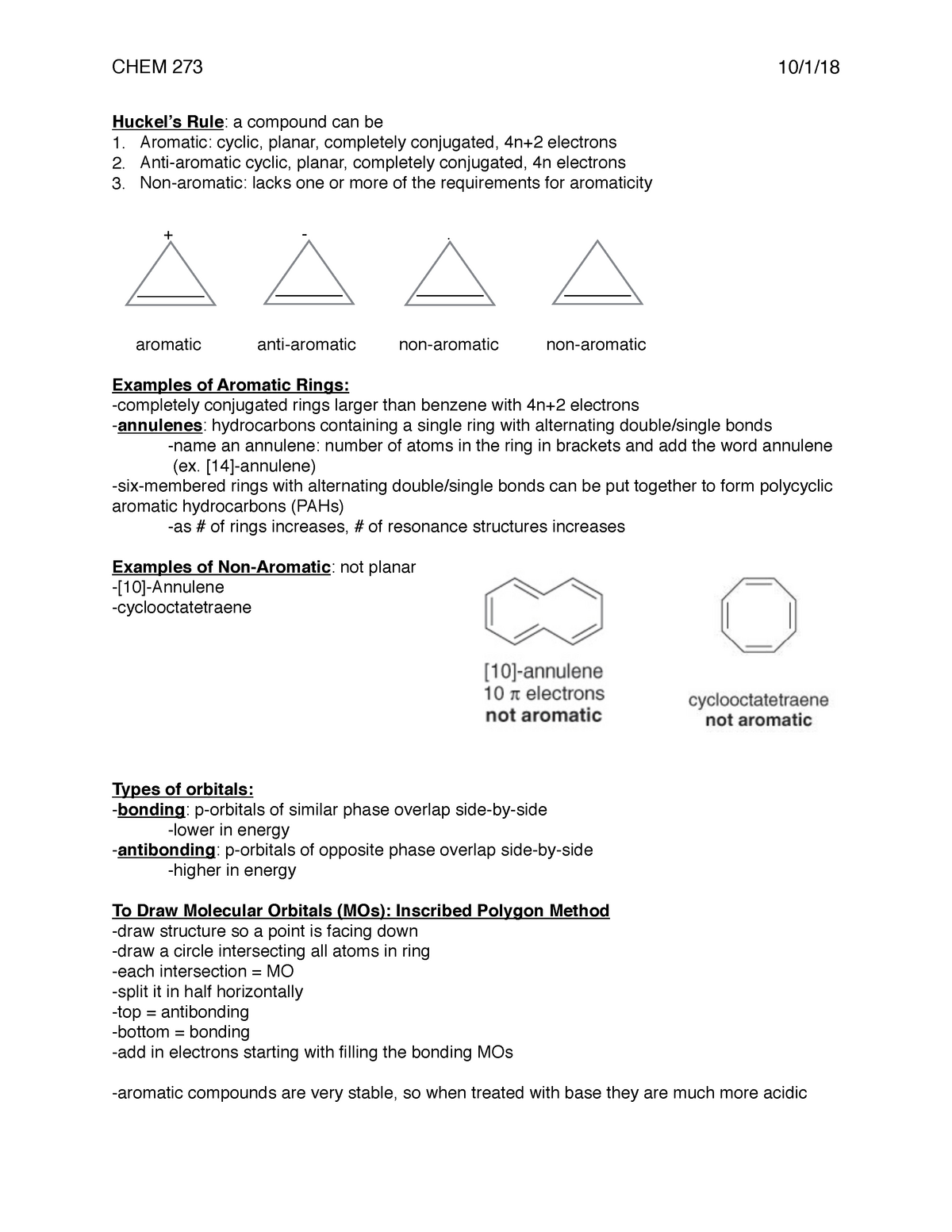



Lecture Notes Aromaticity Rules Chem 273 10 1 18 Huckel S Rule A Compound Can Be 1 Aromatic Studocu




Aromatic Compound Characteristics Examples What Is An Aromatic Compound Video Lesson Transcript Study Com
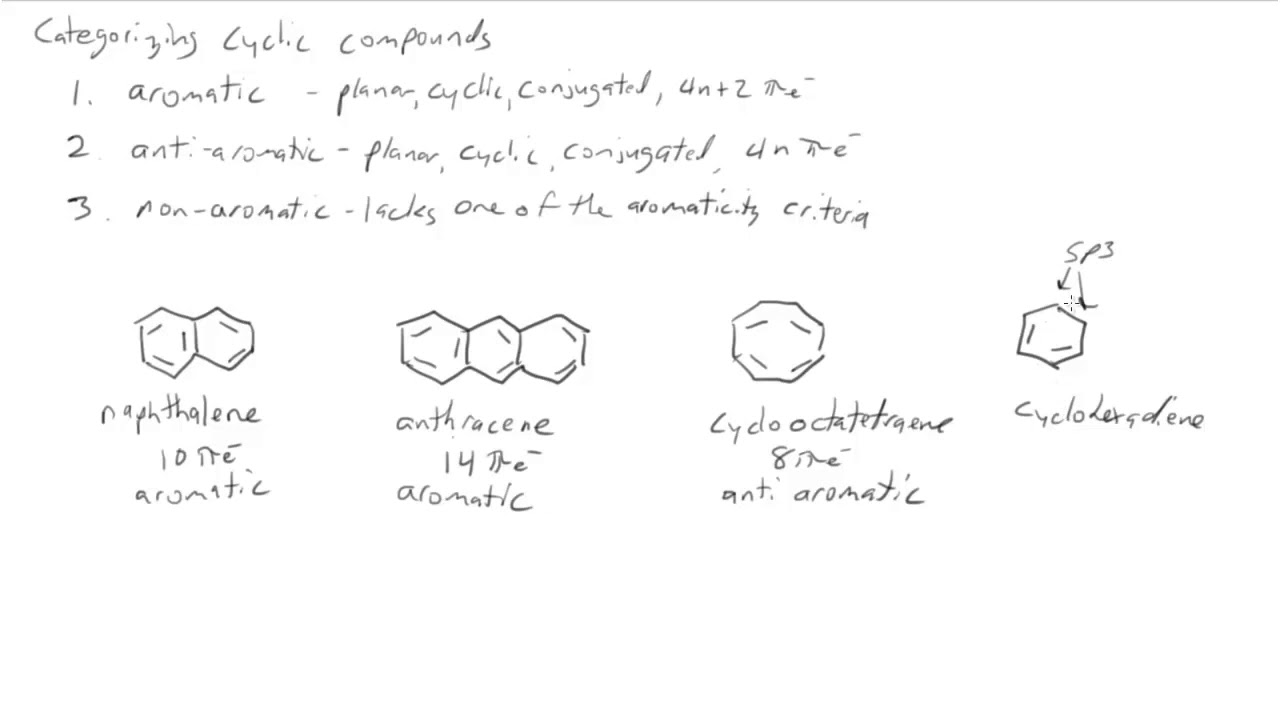



13 7 The Criteria For Aromaticity Huckel S Rule Chemistry Libretexts
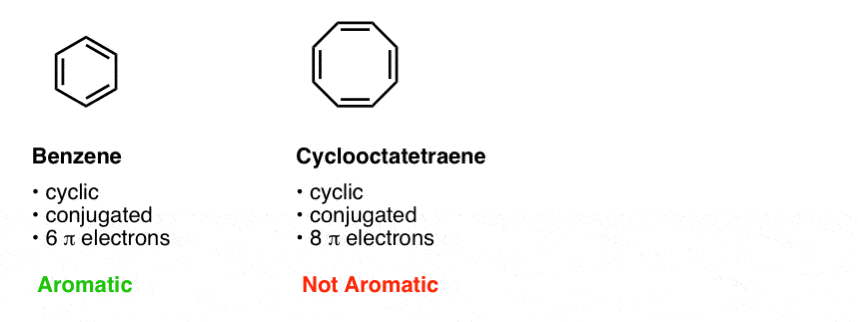



Rules For Aromaticity The 4 Key Factors Master Organic Chemistry
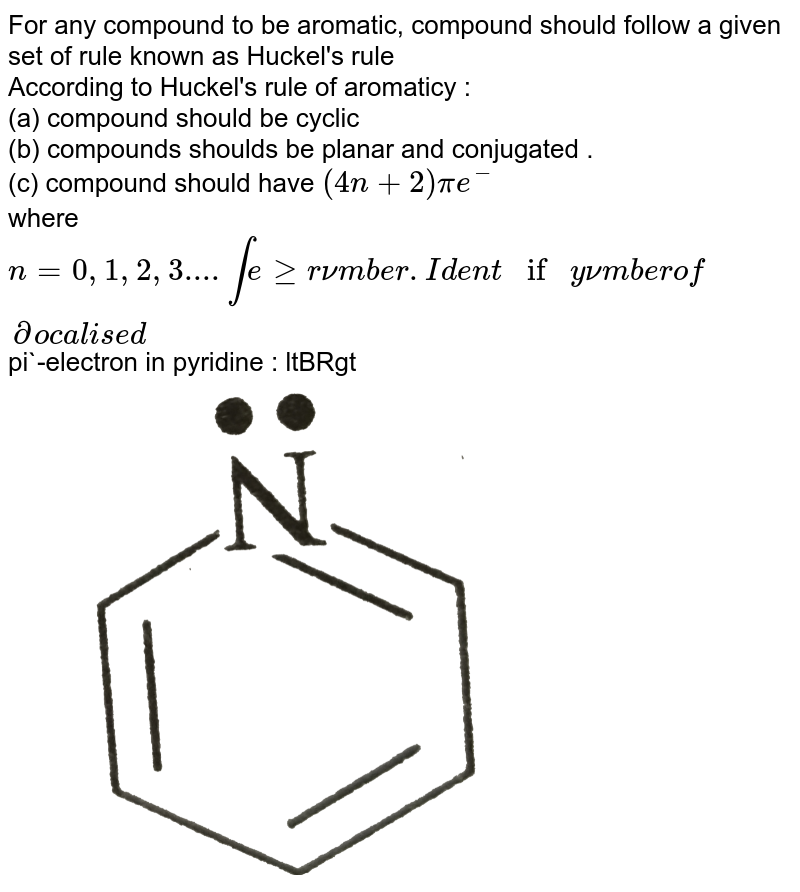



For Any Compound To Be Aromatic Compound Should Follow A Cartain Rule Known As Huckel S Rule According To Huckel S Rule Of Aromaticity A Compound Should Be Cyclic B Compound Should Be Planar



Illustrated Glossary Of Organic Chemistry Term




Aromatic Compound An Overview Sciencedirect Topics
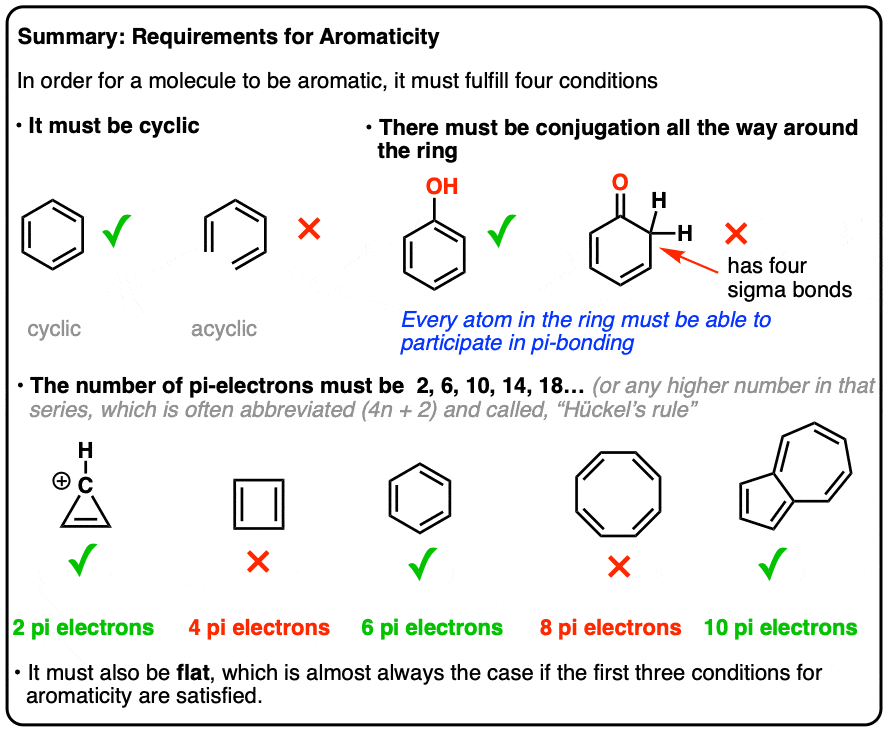



Rules For Aromaticity The 4 Key Factors Master Organic Chemistry




Aromatic Compounds Benzene Its Family Nanoplasmonic Research Group Organic Chemistry Chapter 4 Part I Ppt Download
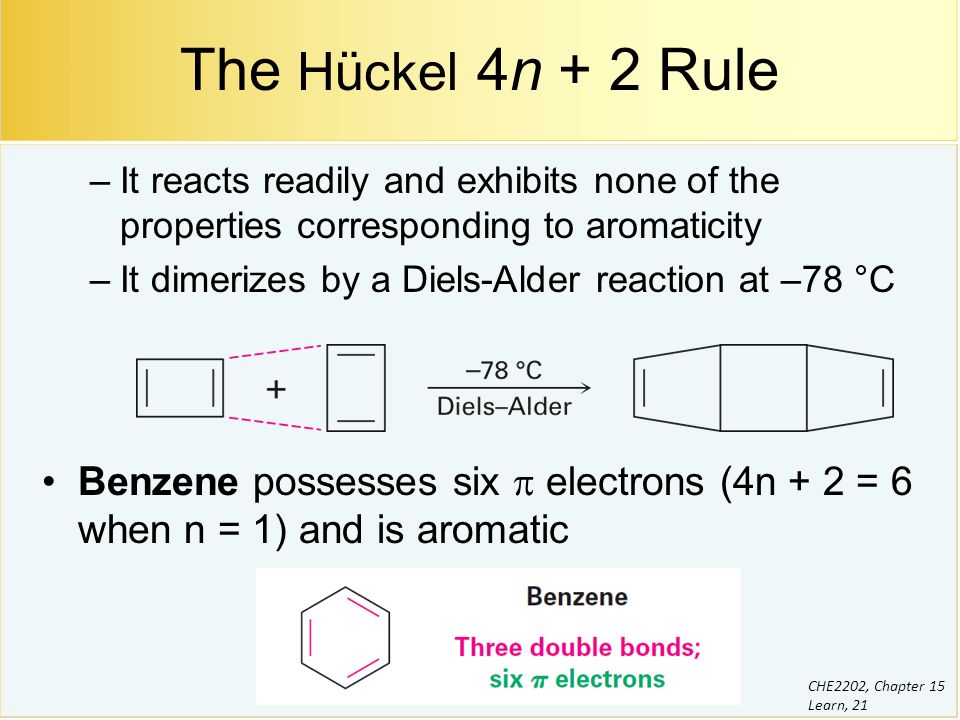



Benzene And Aromaticity Ppt Video Online Download




Huckel S Rule Aromatic And Antiaromatic Compounds Chemistry Steps




Huckel S Rule Explanation Of Huckel S 4n 2 Rule To Estimate Aromaticity
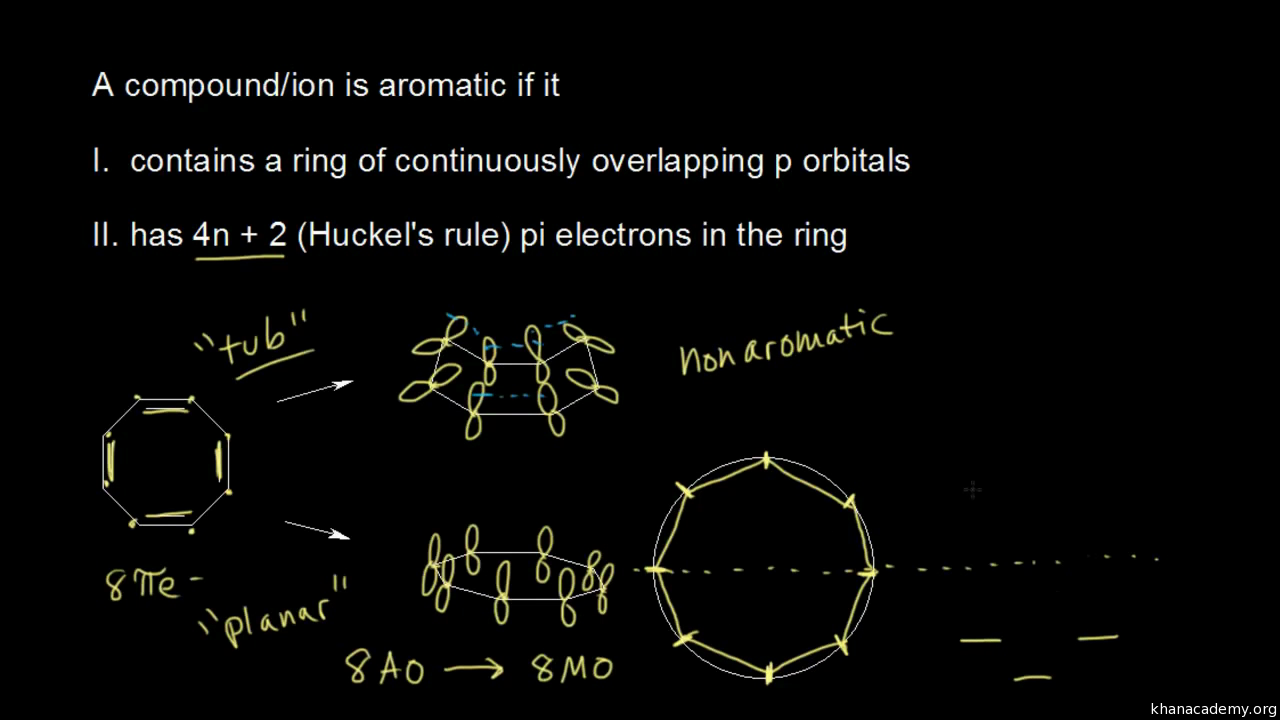



Aromatic Stability Ii Video Khan Academy




Huckel S Rule What Does 4n 2 Mean Master Organic Chemistry
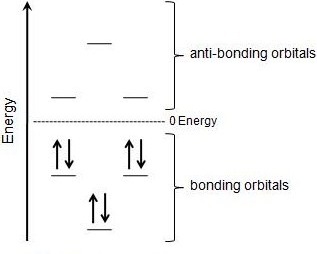.jpg?revision=1&size=bestfit&width=317&height=254)



Huckel S Rule Chemistry Libretexts
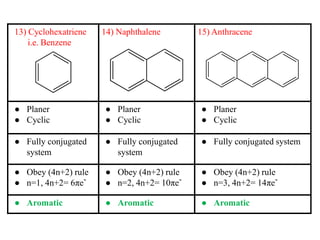



1 Aromaticity



2




Aromatic Antiaromatic Or Nonaromatic Compounds Chemistry Steps



Illustrated Glossary Of Organic Chemistry Term
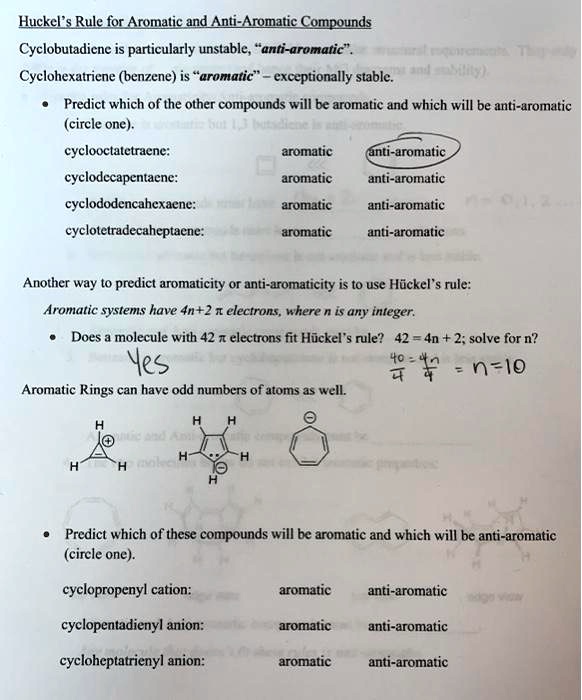



Solved Huckel S Rule For Aromatic And Anti Aromatic Compounds Cyclobutadicne Is Particularly Unstable Anti Aromatic Cyclohexatriene Benzene Is Aromatic Exceptionally Stable Predict Which Of The Other Compounds Will Be Aromatic And Which Will
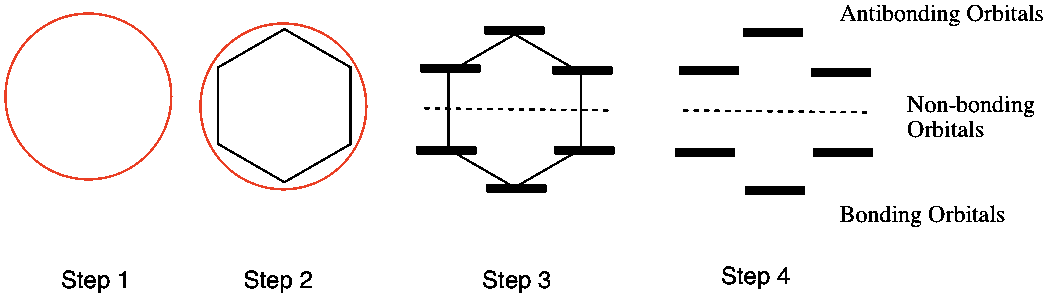



Huckel Aromaticity And Frost Circles



Molecular Orbitals Of A Benzene As A 2d Aromatic Archetype Molecule Download Scientific Diagram
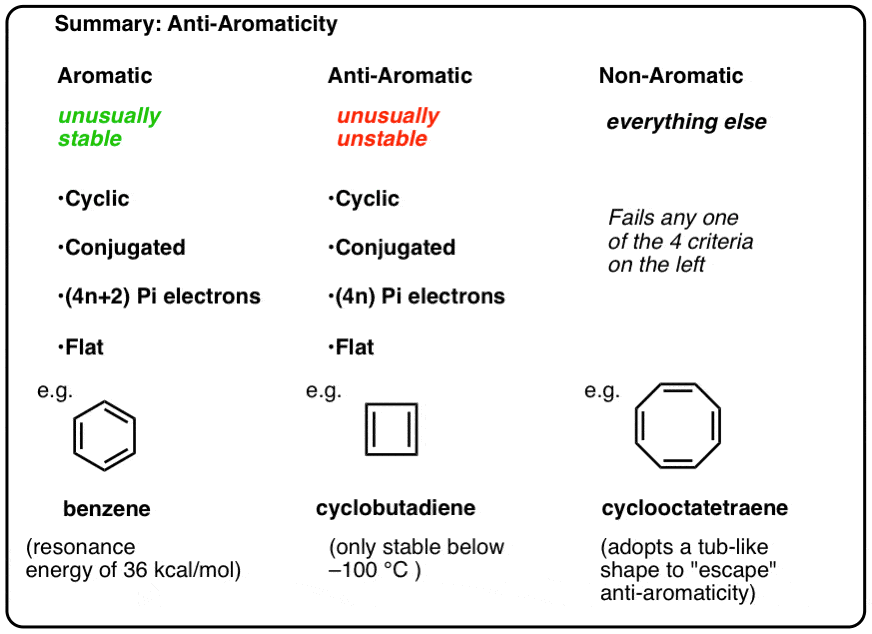



Antiaromaticity And Antiaromatic Compounds Master Organic Chemistry



Q Tbn And9gcqfp0f9alzoqvepqgqpimc6o 1xckjbvgoli5dkqlxhggdlzrvidjd Usqp Cau



Illustrated Glossary Of Organic Chemistry Term
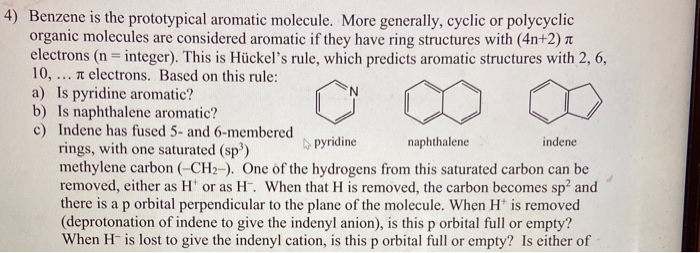



Solved 4 Benzene Is The Prototypical Aromatic Molecule Chegg Com



Organic Chemistry On Line



Illustrated Glossary Of Organic Chemistry Term



Huckel S Rule Explanation Of Huckel S 4n 2 Rule To Estimate Aromaticity



Anti Aromaticity Avoided A Tutorial Example Henry Rzepa S Blog



Aromaticity Rules And Definition



Ppt 15 Benzene And Aromaticity Powerpoint Presentation Free Download Id




Media Portfolio



Aromatic Compounds Definition Example Properties Nomenclature With Videos




13 6 Aromaticity Organic Chemistry Ii




Huckel S Rule Aromatic And Antiaromatic Compounds Chemistry Steps



Q Tbn And9gcszxwzkplc7g Fg Fnpblol Hacooz3xzll6iedlvyaykitxb8hah1h Usqp Cau



Pericyclic Reaction Selection Rules



15 3 Aromaticity And The Huckel 4n 2 Rule Chemistry Libretexts




Aromatic Compounds Ppt Video Online Download



13 6 Aromaticity Chemistry Libretexts



Illustrated Glossary Of Organic Chemistry Term
.jpg?revision=1?readerView)



15 7 The Criteria For Aromaticity Huckel S Rule Chemistry Libretexts




Chapter 15 Benzene And Aromaticity Ppt Download




Criteria For Aromaticity Organic Chemistry Huckel S Rule Benzene Neb Iom Moe Bpkihs Chemistry Youtube



Explain The Huckel Rule With Example




Huckel S Rule Aromatic And Antiaromatic Compounds Chemistry Steps
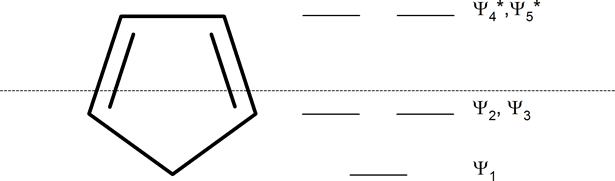



15 3 Aromaticity And The Huckel 4n 2 Rule Chemistry Libretexts



Properties Of Aromatic Compounds Chemistry Revision Site
コメント
コメントを投稿